| Project Name | Project Stage | Molecule Type | Host Species | Therapeutic Area | Indications |
| CD25 mAb - 01 | PCC | Solid Tumor | Solid tumor |
| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| ILA-C52H8 | Cynomolgus | Cynomolgus IL-2 R alpha / CD25 Protein, His Tag (MALS verified) |  |
|

|
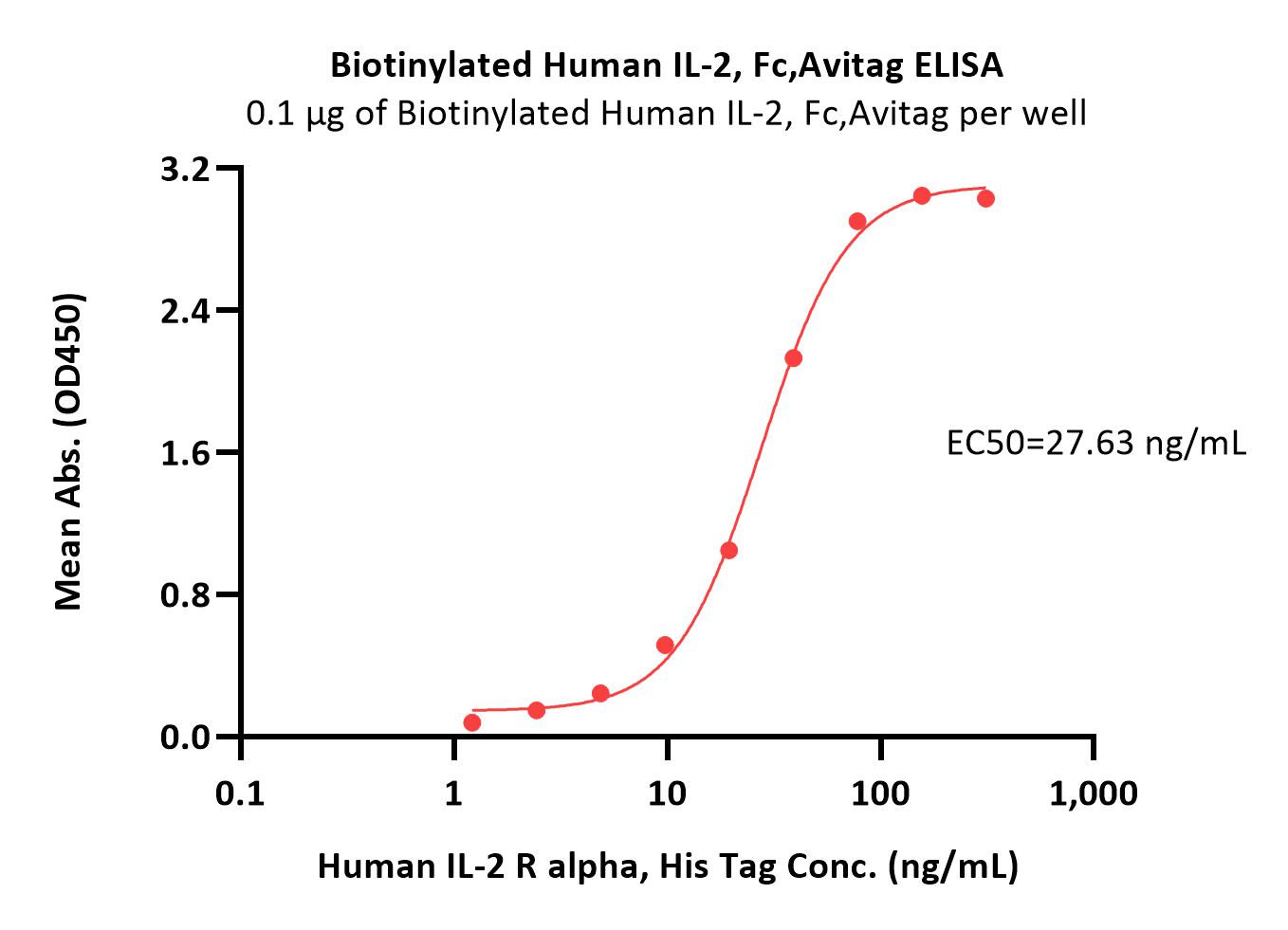
| ILA-H82F9 | Human | Biotinylated Human IL-2 R alpha / CD25 Protein, Fc,Avitag™ (MALS verified) |  |
|
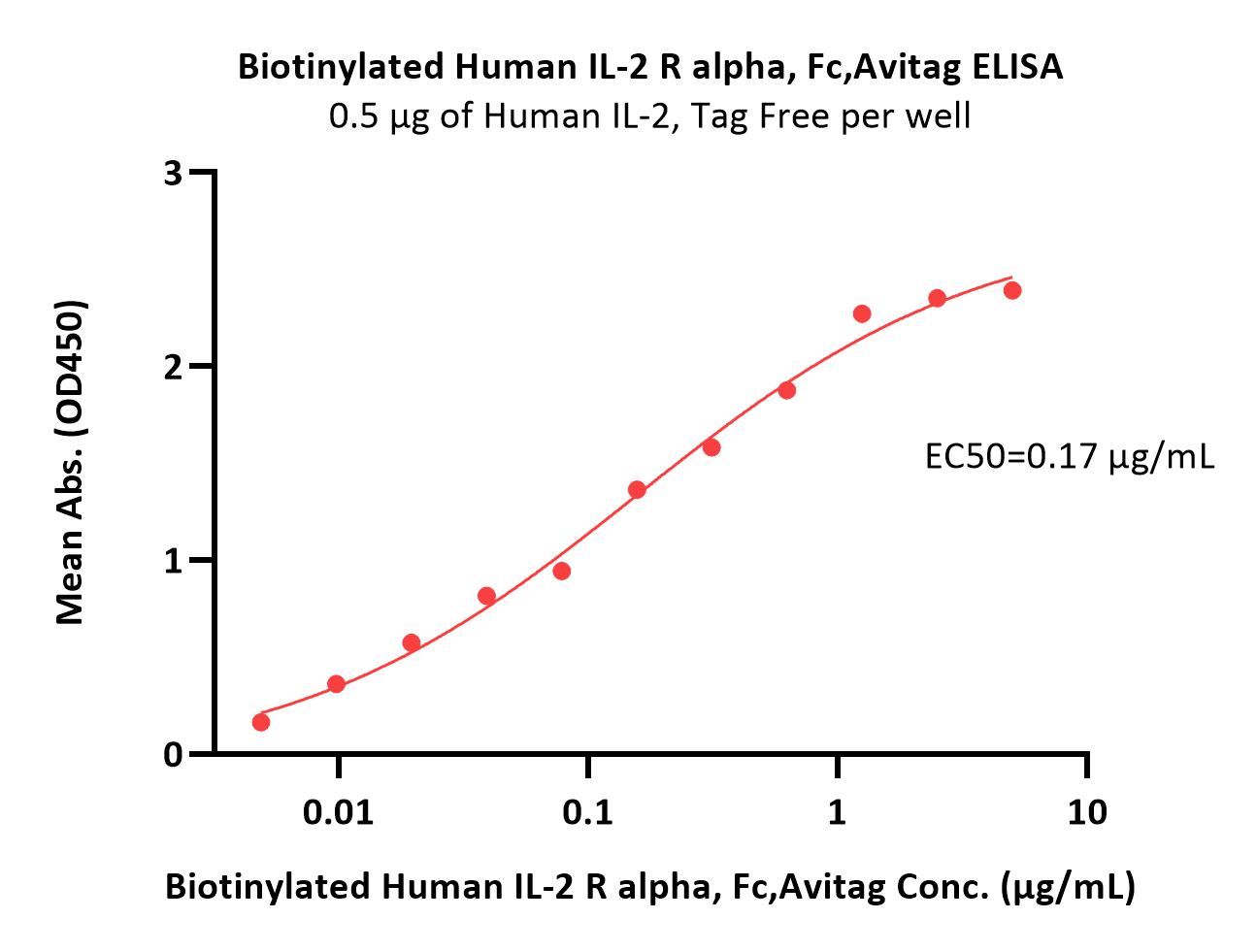
|
| ILA-C52H4 | Canine | Canine IL-2 R alpha / CD25 Protein, His Tag |  |
|
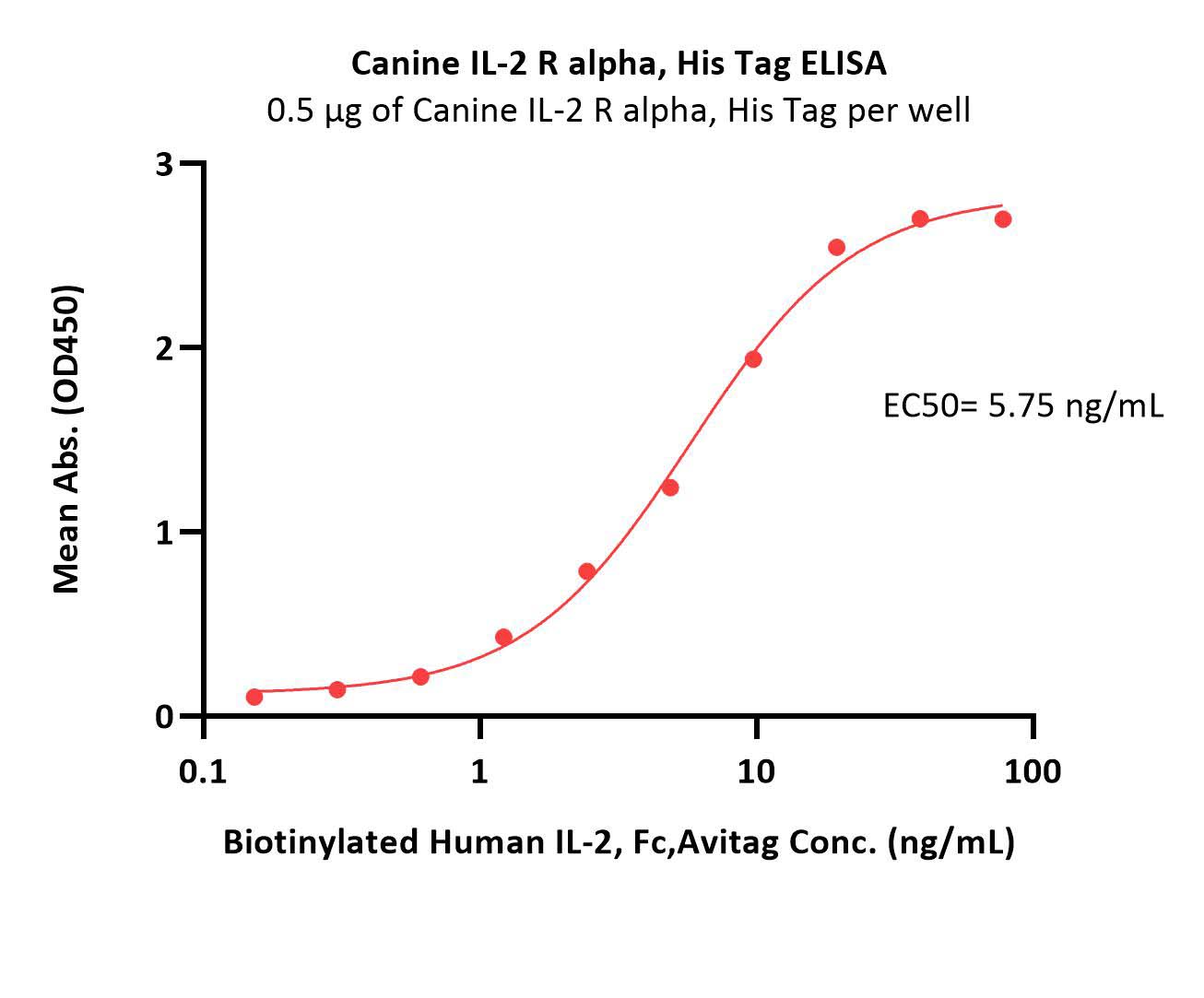
|
| ILA-M52H9 | Mouse | Mouse IL-2 R alpha / CD25 Protein, His Tag |  |
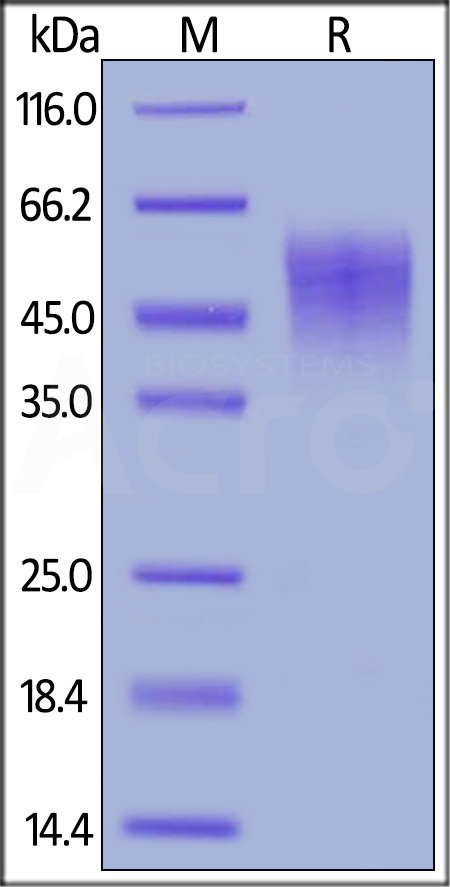
|

|
| ILA-H82E6 | Human | Biotinylated Human IL-2 R alpha / CD25 Protein, His,Avitag™, premium grade |  |
|

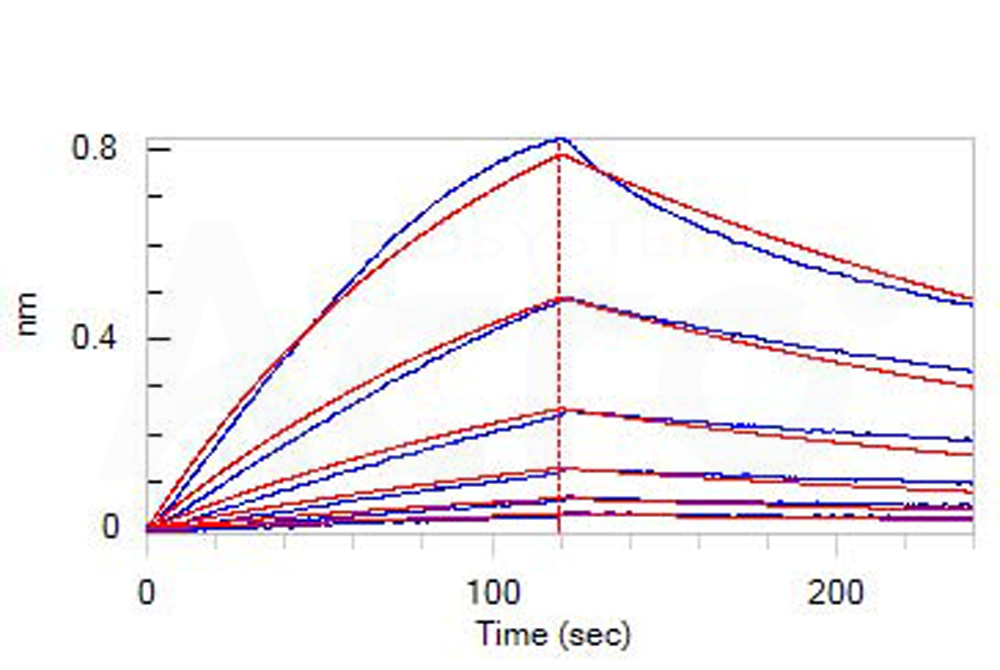
|
| ILA-H52H9 | Human | Human IL-2 R alpha / CD25 Protein, His Tag |  |
|

|
| ILA-H5251 | Human | Human IL-2 R alpha / CD25 Protein, Fc Tag (MALS verified) |  |

|
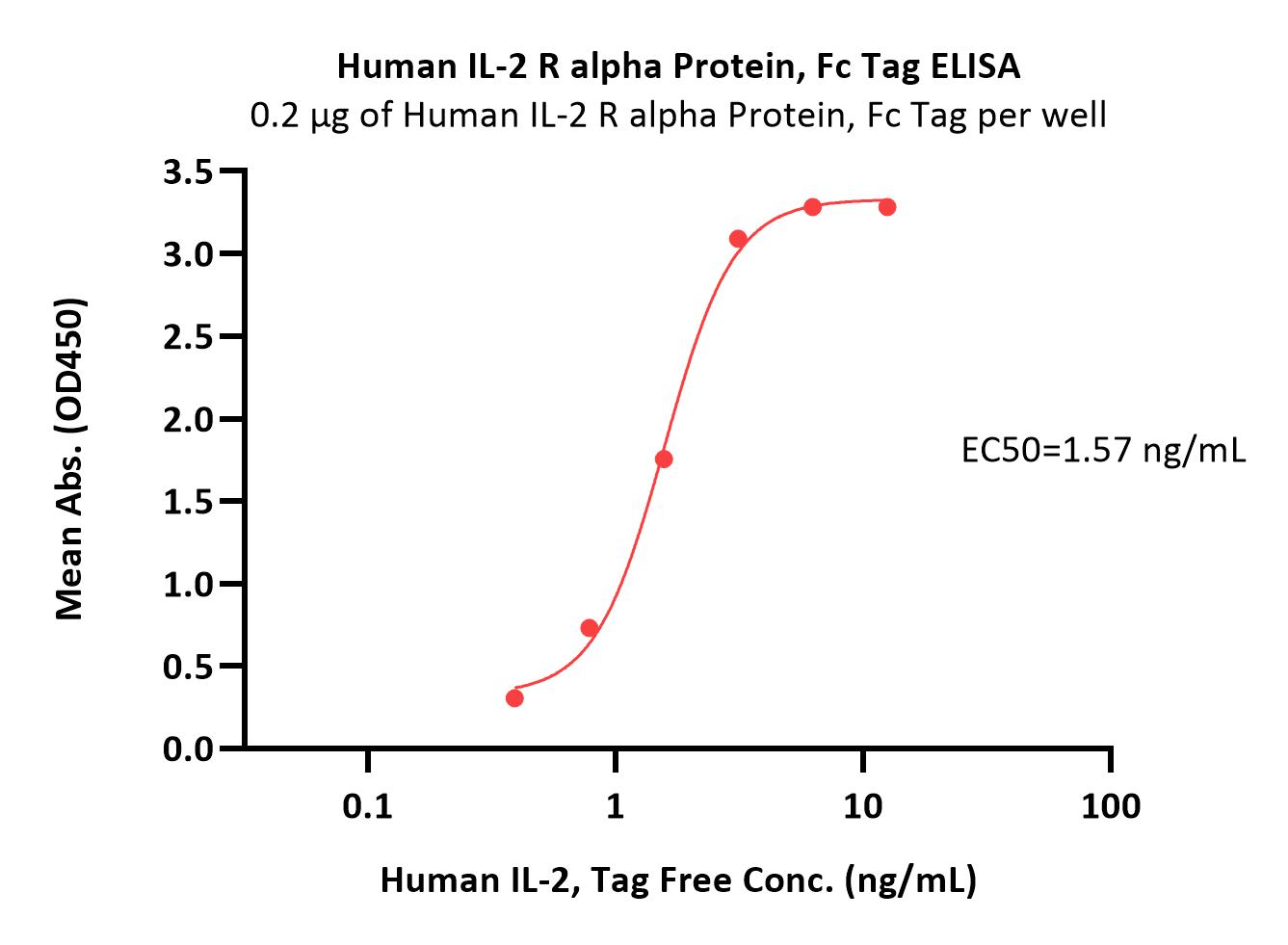
|

Biotinylated Human IL-2 R alpha, His,Avitag, premium grade (Cat. No. ILA-H82E6) inhibits the IL-2-dependent proliferation of Mo7e cells. The EC50 for this effect is 0.57-0.81 µg/mL (Routinely tested).

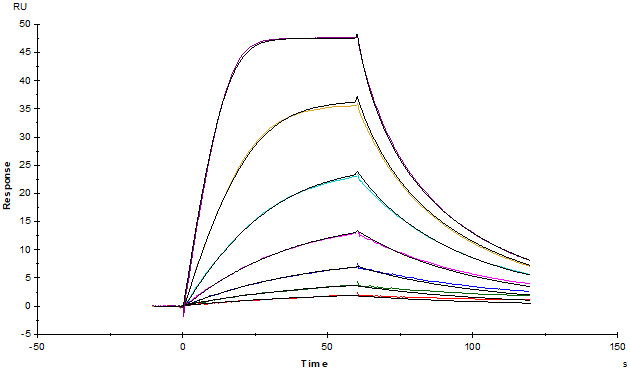
Human IL-2 R alpha, His Tag (Cat. No. ILA-H52H9) captured on CM5 chip via anti-His antibody, can bind Human IL-2, Tag Free with an affinity constant of 29.9 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Daclizumab biosimilar (Shanghai CP Guojian) | Approved | Shanghai Cp Guojian Pharmaceutical Co Ltd | 健尼哌 | Mainland China | Rejection of renal transplantation | null | 2011-01-12 | Rejection of renal transplantation | Details | |
| Basiliximab | CHT-25; chRFT5; CHI-621; SDZ-CHI-621 | Approved | Novartis Pharma Ag | 舒莱, Simulect | Japan | Rejection of organ transplantation | Novartis Pharma Ag | 1998-05-12 | Anemia, Aplastic; Leukemia, Lymphocytic, Chronic, B-Cell; Kidney Failure, Chronic; Uveitis; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin; Emphysema; Pulmonary Disease, Chronic Obstructive; Colitis, Ulcerative; Primary Myelofibrosis; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Rejection of renal transplantation; Multiple Myeloma; Rejection of organ transplantation; Hodgkin Disease; Coronavirus Disease 2019 (COVID-19); Myelodysplastic Syndromes; Keratoplasty rejection; Hemoglobinuria, Paroxysmal; Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Cytokine Release Syndrome | Details |
| Daclizumab | R-35; BIIB-019; RO-247375 | Approved | Abbvie Inc, Biogen Inc, F. Hoffmann-La Roche Ltd | Zinbryta, Zenapax | United States | Multiple Sclerosis | Biogen Inc | 1997-12-10 | Retinal Diseases; Leukemia, T-Cell; Behcet Syndrome; Melanoma; Iritis; Thrombocytopenia; Uveitis; Rejection in heart transplantation; Lymphoma; Uveitis, Anterior; Colitis, Ulcerative; Asthma; Breast Neoplasms; Psoriasis; Multiple Sclerosis, Relapsing-Remitting; Arthritis, Juvenile; Radiation Injuries; Carcinoma, Ductal; Rejection of organ transplantation; Multiple Sclerosis; Myelodysplastic Syndromes; HTLV-I Infections; Gastrointestinal Diseases; Inflammatory Bowel Diseases; Granulomatosis with Polyangiitis; Diabetes Mellitus, Type 1; Leukemia; HIV Infections | Details |
| Daclizumab biosimilar (Shanghai CP Guojian) | Approved | Shanghai Cp Guojian Pharmaceutical Co Ltd | 健尼哌 | Mainland China | Rejection of renal transplantation | null | 2011-01-12 | Rejection of renal transplantation | Details | |
| Basiliximab | CHT-25; chRFT5; CHI-621; SDZ-CHI-621 | Approved | Novartis Pharma Ag | 舒莱, Simulect | Japan | Rejection of organ transplantation | Novartis Pharma Ag | 1998-05-12 | Anemia, Aplastic; Leukemia, Lymphocytic, Chronic, B-Cell; Kidney Failure, Chronic; Uveitis; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin; Emphysema; Pulmonary Disease, Chronic Obstructive; Colitis, Ulcerative; Primary Myelofibrosis; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Rejection of renal transplantation; Multiple Myeloma; Rejection of organ transplantation; Hodgkin Disease; Coronavirus Disease 2019 (COVID-19); Myelodysplastic Syndromes; Keratoplasty rejection; Hemoglobinuria, Paroxysmal; Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Cytokine Release Syndrome | Details |
| Daclizumab | R-35; BIIB-019; RO-247375 | Approved | Abbvie Inc, Biogen Inc, F. Hoffmann-La Roche Ltd | Zinbryta, Zenapax | United States | Multiple Sclerosis | Biogen Inc | 1997-12-10 | Retinal Diseases; Leukemia, T-Cell; Behcet Syndrome; Melanoma; Iritis; Thrombocytopenia; Uveitis; Rejection in heart transplantation; Lymphoma; Uveitis, Anterior; Colitis, Ulcerative; Asthma; Breast Neoplasms; Psoriasis; Multiple Sclerosis, Relapsing-Remitting; Arthritis, Juvenile; Radiation Injuries; Carcinoma, Ductal; Rejection of organ transplantation; Multiple Sclerosis; Myelodysplastic Syndromes; HTLV-I Infections; Gastrointestinal Diseases; Inflammatory Bowel Diseases; Granulomatosis with Polyangiitis; Diabetes Mellitus, Type 1; Leukemia; HIV Infections | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Basiliximab biobetter (Mabtech/Sorrento) | STI-003 | Phase 3 Clinical | Taizhou Maibo Taike Biotechnology Co Ltd | Autoimmune Diseases | Details |
| MDNA-11 | MDNA-11; MDNA11 | Phase 2 Clinical | Medicenna Therapeutics Corp | Solid tumours | Details |
| RM-1995 | RM-1995 | Rakuten Medical Inc | Squamous Cell Carcinoma of Head and Neck; Carcinoma, Squamous Cell | Details | |
| Inolimomab | B-B10; BT-563; MAb-BT-563 | Eusa Pharma, Jazz Pharmaceuticals Plc | Details | ||
| Anti-interleukin-2 receptor monoclonal antibody Mikbeta1 | HuMikβ1-1 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd, National Institutes Of Health | Leukemia; Leukemia, Lymphoid; Celiac Disease; Paraparesis, Tropical Spastic; Leukemia, Large Granular Lymphocytic | Details |
| LMB-2 | LMB-2; LMB-2a | Phase 2 Clinical | National Cancer Institute | Leukemia; Leukemia, Hairy Cell; Skin Neoplasms; Myelodysplastic Syndromes; Myeloproliferative Disorders; Leukemia-Lymphoma, Adult T-Cell; Lymphoma; Melanoma; Myelodysplastic-Myeloproliferative Diseases | Details |
| 90Y Basiliximab | Phase 2 Clinical | City Of Hope National Medical Center | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, T-Cell, Cutaneous; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell | Details | |
| In 111-DOTA-Basiliximab | Phase 1 Clinical | City Of Hope National Medical Center | Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details | |
| RO-7296682 | RO-7296682; RG-6292 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms | Details |
| Nemvaleukin alfa | RDB-1450; RDB-1419; ALKS-4230 | Phase 3 Clinical | Alkermes Plc | Solid tumours; Ovarian Neoplasms; Skin Melanoma; Squamous Cell Carcinoma of Head and Neck; Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Melanoma | Details |
| PT-101 | PT-101; MK-6194 | Phase 1 Clinical | Pandion Therapeutics | Colitis, Ulcerative; Dermatitis, Atopic | Details |
| XmAb-564 | XmAb-564; XmAb-27564 | Phase 1 Clinical | Xencor Inc | Autoimmune Diseases | Details |
| BA-1106 | TS-1904; BA-1106; RR-102 | Phase 1 Clinical | Shandong Boan Biotechnology Co Ltd | Solid tumours | Details |
| Camidanlumab tesirine | ADCT-301 | Phase 2 Clinical | Genmab A/S, Adc Therapeutics Sa | Myelodysplastic Syndromes; Melanoma; Carcinoma, Non-Small-Cell Lung; Lymphoma, Non-Hodgkin; Leukemia, Myeloid, Acute; Fallopian Tube Neoplasms; Colorectal Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Urinary Bladder Neoplasms; Myeloproliferative Disorders; Solid tumours; Hodgkin Disease; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms; Ovarian Neoplasms | Details |
| Basiliximab biobetter (Mabtech/Sorrento) | STI-003 | Phase 3 Clinical | Taizhou Maibo Taike Biotechnology Co Ltd | Autoimmune Diseases | Details |
| MDNA-11 | MDNA-11; MDNA11 | Phase 2 Clinical | Medicenna Therapeutics Corp | Solid tumours | Details |
| RM-1995 | RM-1995 | Rakuten Medical Inc | Squamous Cell Carcinoma of Head and Neck; Carcinoma, Squamous Cell | Details | |
| Inolimomab | B-B10; BT-563; MAb-BT-563 | Eusa Pharma, Jazz Pharmaceuticals Plc | Details | ||
| Anti-interleukin-2 receptor monoclonal antibody Mikbeta1 | HuMikβ1-1 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd, National Institutes Of Health | Leukemia; Leukemia, Lymphoid; Celiac Disease; Paraparesis, Tropical Spastic; Leukemia, Large Granular Lymphocytic | Details |
| LMB-2 | LMB-2; LMB-2a | Phase 2 Clinical | National Cancer Institute | Leukemia; Leukemia, Hairy Cell; Skin Neoplasms; Myelodysplastic Syndromes; Myeloproliferative Disorders; Leukemia-Lymphoma, Adult T-Cell; Lymphoma; Melanoma; Myelodysplastic-Myeloproliferative Diseases | Details |
| 90Y Basiliximab | Phase 2 Clinical | City Of Hope National Medical Center | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, T-Cell, Cutaneous; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell | Details | |
| In 111-DOTA-Basiliximab | Phase 1 Clinical | City Of Hope National Medical Center | Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details | |
| RO-7296682 | RO-7296682; RG-6292 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms | Details |
| Nemvaleukin alfa | RDB-1450; RDB-1419; ALKS-4230 | Phase 3 Clinical | Alkermes Plc | Solid tumours; Ovarian Neoplasms; Skin Melanoma; Squamous Cell Carcinoma of Head and Neck; Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Melanoma | Details |
| PT-101 | PT-101; MK-6194 | Phase 1 Clinical | Pandion Therapeutics | Colitis, Ulcerative; Dermatitis, Atopic | Details |
| XmAb-564 | XmAb-564; XmAb-27564 | Phase 1 Clinical | Xencor Inc | Autoimmune Diseases | Details |
| BA-1106 | TS-1904; BA-1106; RR-102 | Phase 1 Clinical | Shandong Boan Biotechnology Co Ltd | Solid tumours | Details |
| Camidanlumab tesirine | ADCT-301 | Phase 2 Clinical | Genmab A/S, Adc Therapeutics Sa | Myelodysplastic Syndromes; Melanoma; Carcinoma, Non-Small-Cell Lung; Lymphoma, Non-Hodgkin; Leukemia, Myeloid, Acute; Fallopian Tube Neoplasms; Colorectal Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Urinary Bladder Neoplasms; Myeloproliferative Disorders; Solid tumours; Hodgkin Disease; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms; Ovarian Neoplasms | Details |
This web search service is supported by Google Inc.
